

ISO 13485:2016 certification ensures top-quality medical devices, regulatory compliance, and global trust through effective QMS implementation.
In today’s dynamic healthcare environment, quality is non-negotiable. Organizations involved in designing, producing, and distributing medical devices must maintain the highest quality and regulatory standards. This is where ISO 13485:2016 Medical Devices Certification plays a pivotal role. It provides a globally recognized framework to establish and maintain an effective quality management system tailored for the medical device industry.
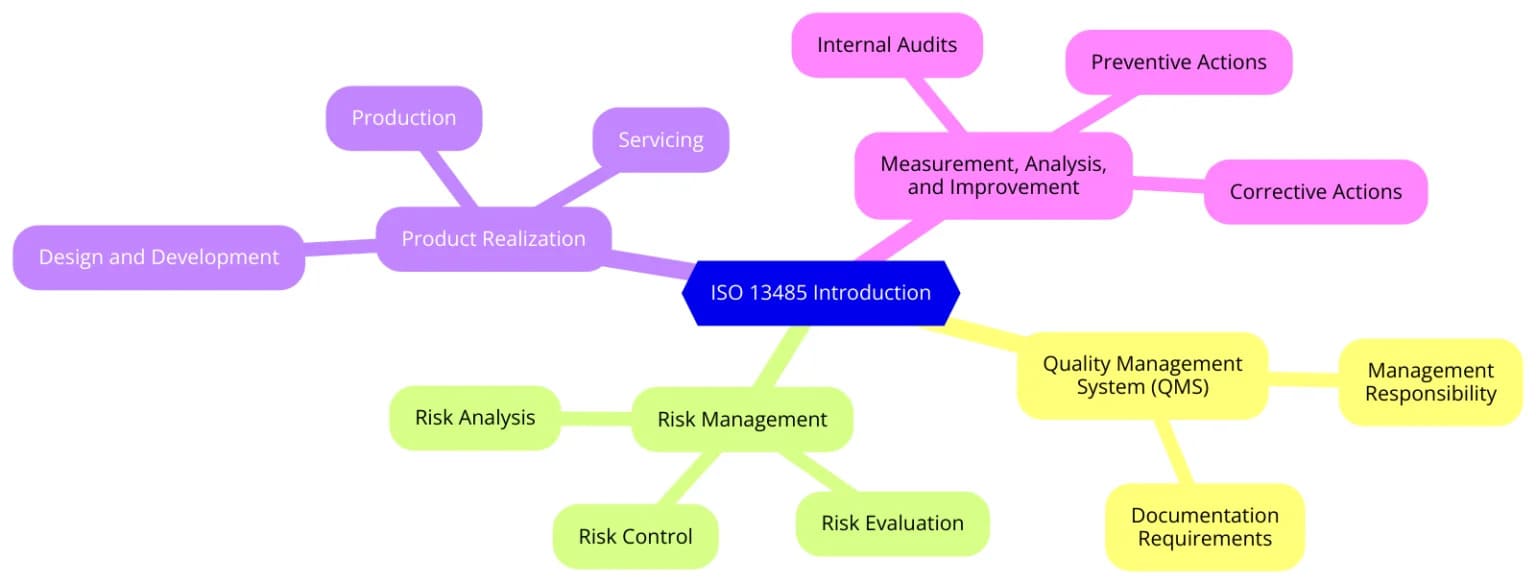
ISO 13485:2016 outlines the requirements for a quality management system specific to medical devices. It supports regulatory compliance, improves risk management, and ensures consistent product quality. Aligning with this standard builds trust with regulators, partners, and patients.
It also helps organizations conduct a fair certification service ISO 13485 2016 validity check—critical for ensuring continued compliance and avoiding penalties.
Gaining ISO 13485:2016 Medical Devices Certification demonstrates a manufacturer’s commitment to patient safety and product performance. It’s not just about meeting requirements—it’s about standing out in a highly competitive market.
When integrated effectively, this system brings structure to internal processes, reduces waste, and enhances customer satisfaction. Companies can also perform an accurate fair certification service ISO 13485 2016 validity check to verify the legitimacy of their certificate and auditors.
Getting certified is a multi-phase process that includes:
Through this structured path, your organization moves closer to achieving ISO 13485:2016 Medical Devices Certification and gaining competitive advantage.
Organizations must maintain their QMS post-certification through surveillance audits. It’s also necessary to undergo recertification every three years. A fair certification service ISO 13485 2016 validity check ensures that your certification remains active, valid, and recognized by authorities.
Staying current with the iso13485 2016 version allows companies to adapt to changing regulatory landscapes while maintaining operational efficiency.
The benefits of ISO 13485:2016 Medical Devices Certification are long-lasting:
Incorporating iso 13485 2016 certification requirements ensures that businesses are not just compliant, but also proactive in driving excellence.
In a world where safety and quality determine success in healthcare, ISO 13485:2016 Medical Devices Certification stands as a critical asset. Whether you are a start-up or an established manufacturer, this certification can transform your operations and boost your market presence.
By adopting iso13485 2016 standards, integrating effective QMS practices, and using a fair certification service ISO 13485 2016 validity check, organizations can achieve long-term sustainability and patient-centric excellence.
ISO 13485 is used to set up and maintain a quality management system specifically for medical device manufacturing and services. It ensures that organizations can consistently meet customer and regulatory requirements.
No, ISO 13485:2016 Medical Devices Certification is not legally mandatory, but it is often required by international markets and customers. It also aligns with regulatory frameworks in many regions.
The benefits include improved product quality, enhanced customer satisfaction, global market access, reduced compliance risks, and more efficient internal processes aligned with iso 13485 2016 certification.
Typically, ISO 13485:2016 Medical Devices Certification is valid for three years, subject to annual surveillance audits. A fair certification service ISO 13485 2016 validity check helps confirm active compliance throughout this period.
While both are quality standards, ISO 13485:2016 is specifically tailored for medical devices and includes regulatory requirements, whereas ISO 9001 is a generic quality management system standard.
Contact us: Pacific Certifications
